Author disclosure: No relevant financial affiliations.
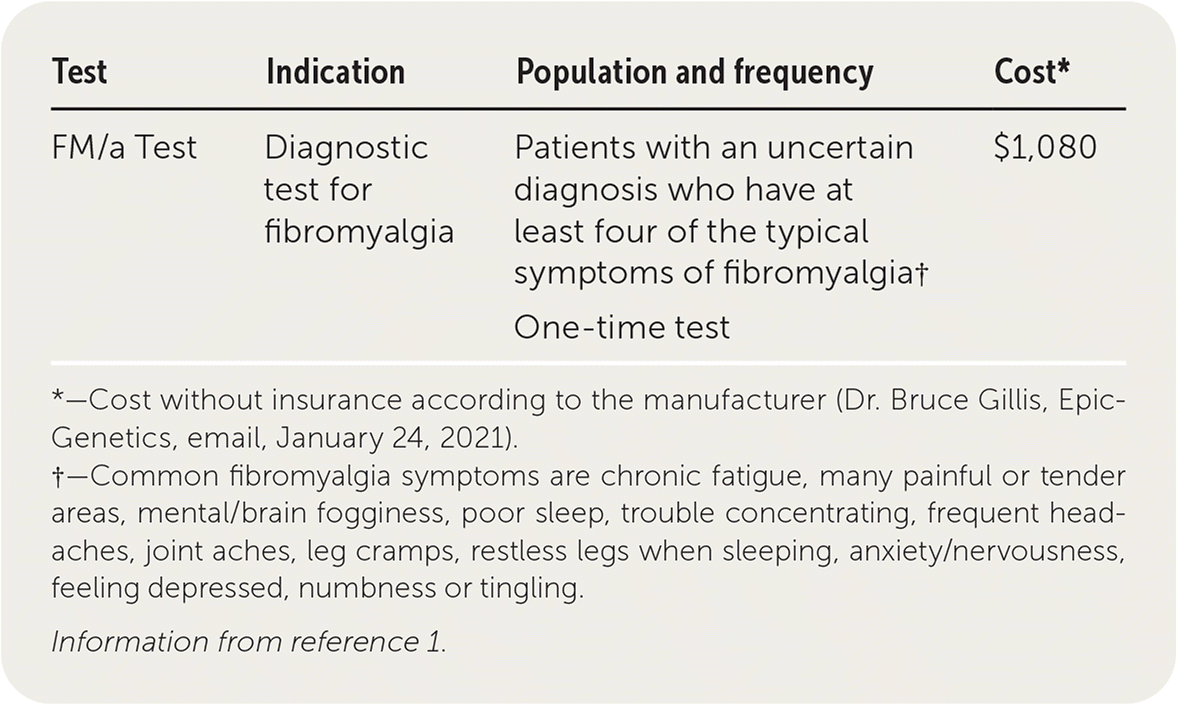
The FM/a Test is a blood test used for the diagnosis of fibromyalgia. It is available for use in patients of any age who have at least four of the typical symptoms of fibromyalgia.1
Accuracy
Fibromyalgia has historically been diagnosed using the American College of Rheumatology clinical criteria (Table 1).2 The FM/a Test is a cytokine assay of in vitro stimulated peripheral blood mononuclear cells. Production of cytokines by stimulated immune cells in patients with fibromyalgia has been shown to be significantly different from that of healthy control patients.3,4 Based on the concentrations of four cytokines, a cytokine/chemokine composite score, calculated as 1 / (1 + e−x) * 100, on a scale of 0 to 100 was developed. A score greater than 50 is considered positive for fibromyalgia.4
TABLE 1.
2016 American College of Rheumatology Fibromyalgia Diagnostic Criteria
zoom_out_mapEnlargeprintPrint
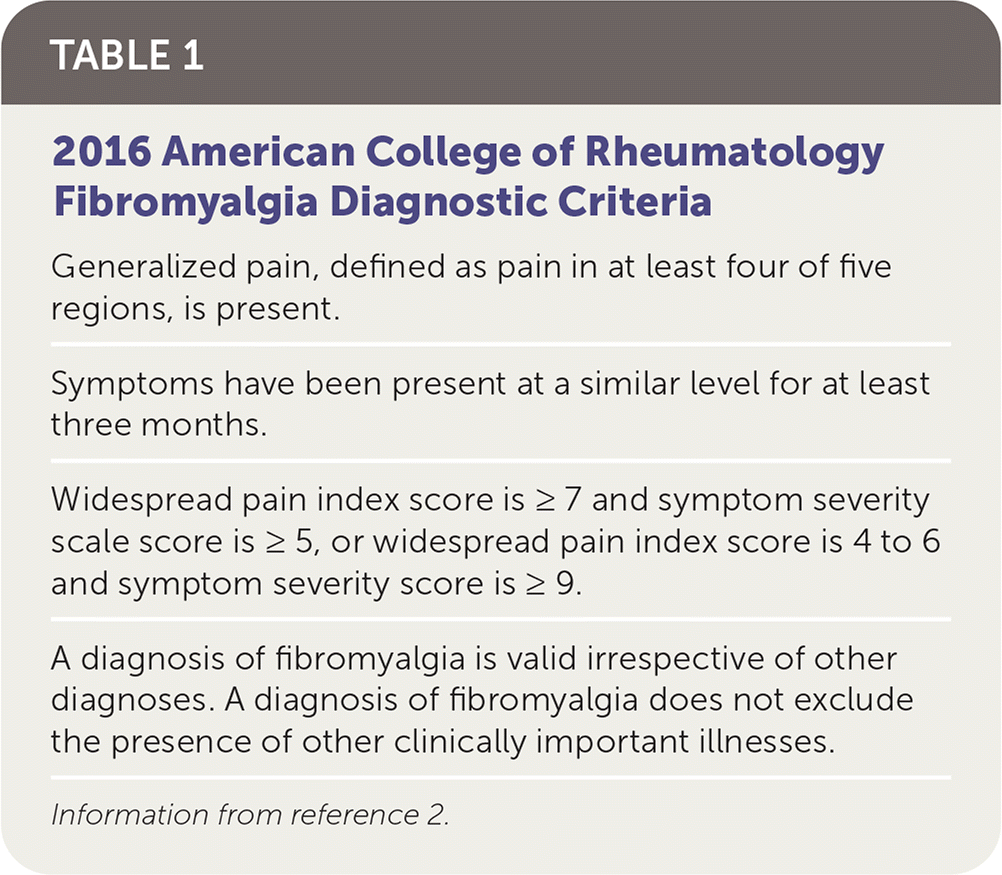
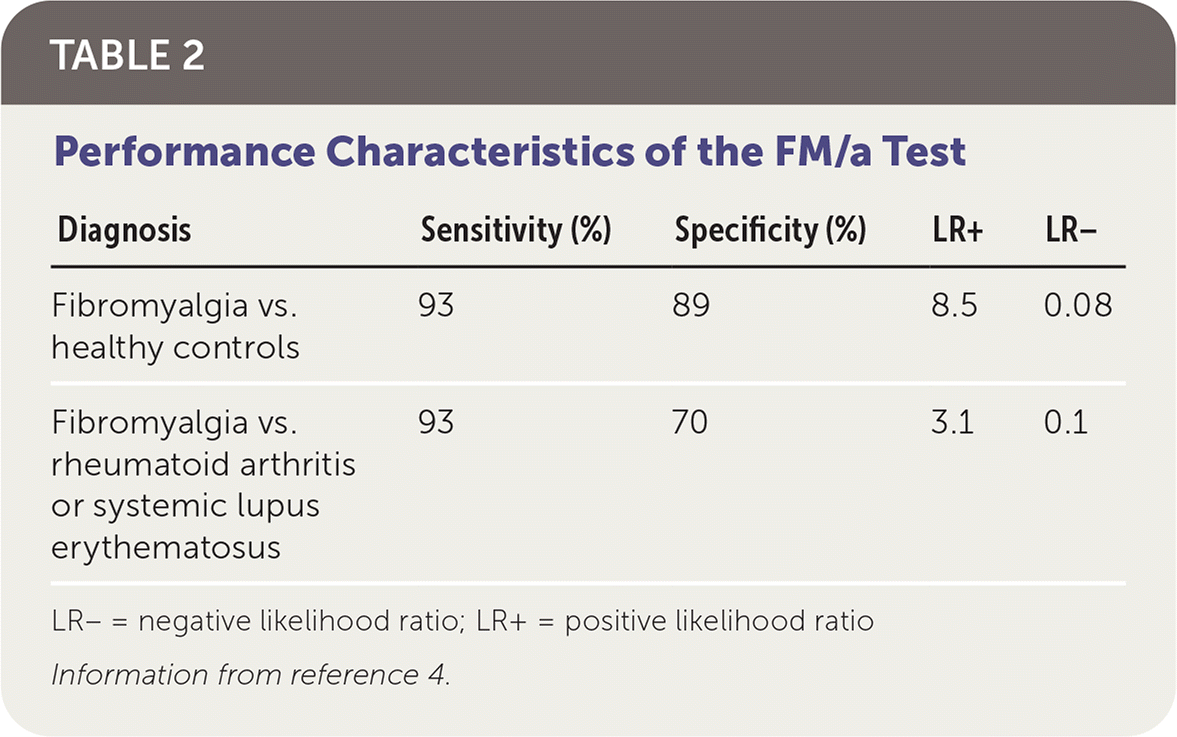
A study of 160 patients with fibromyalgia based on the American College of Rheumatology criteria and 119 healthy control patients found that a positive FM/a Test result has a sensitivity of 93% and a specificity of 89% (Table 2).4 The positive likelihood ratio was 8.5, and the negative likelihood ratio was 0.08. However, this type of study design, which uses a control group of healthy patients, overestimates accuracy. In the same study, when using a more appropriate comparison group of patients with known rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE) who did not have co-occurring fibromyalgia, the specificity was only 70%, with a positive likelihood ratio of 3.1 and a negative likelihood ratio of 0.1. The most appropriate study design would enroll patients with clinically suspected fibromyalgia, but this type of study has not been performed.
TABLE 2.
Performance Characteristics of the FM/a Test
zoom_out_mapEnlargeprintPrint
Benefit
In primary care settings, fibromyalgia is diagnosed inconsistently using the clinical criteria.5 The FM/a Test could help to improve appropriate management and avoid mismanagement. However, there are no studies demonstrating whether the FM/a Test changes management or improves patient outcomes.
Harms
Patients must stop taking any medications that may alter their immune system for the 14 days before administration of the FM/a Test. These include all immunomodulatory agents (e.g., anticancer agents, disease-modifying antirheumatic drugs, steroids, montelukast [Singulair], immunosuppressive agents, and some over-the-counter supplements such as turmeric).1,3 Overdiagnosis or underdiagnosis may be another potential harm because the diagnostic accuracy of the test in the general population has yet to be evaluated. So far, the FM/a Test has only been shown to help differentiate patients with fibromyalgia from those with RA or SLE or from healthy control patients. It has not been shown to distinguish fibromyalgia from nonfibromyalgia in previously undiagnosed patients with several typical fibromyalgia symptoms. Additionally, the false-positive rate of the test was higher in patients with SLE and RA, at 29% and 31%, respectively.4 Because fibromyalgia often co-occurs with autoimmune disorders such as RA and SLE, a positive test result should not preclude an autoimmune workup if clinically indicated.6,7
Cost
According to the manufacturer (Dr. Bruce Gillis, EpicGenetics, email, January 24, 2021), the cost of the FM/a Test without insurance coverage is $1,080. However, many insurance carriers reimburse for the cost of the FM/a Test. It is important to note that earlier diagnosis of fibromyalgia results in cost savings over time (from a decrease in diagnostic testing and medication costs).8
Bottom Line
The FM/a Test is the first laboratory test for the diagnosis of fibromyalgia and is covered by many insurance plans. Limited data suggest that the test accurately differentiates patients with fibromyalgia from healthy controls and has some ability to differentiate fibromyalgia from other rheumatologic diseases such as RA and SLE. However, there is currently no research evaluating the accuracy of the test in the general population or demonstrating any change in patient outcomes.
expand_moreAuthor Information
Address correspondence to Anne Mounsey, MD, at [email protected]. Reprints are not available from the authors.
Author disclosure: No relevant financial affiliations.
expand_moreReference(s)
- EpicGenetics. FM/a Test. Accessed January 23, 2021. http://www.fmtest.com